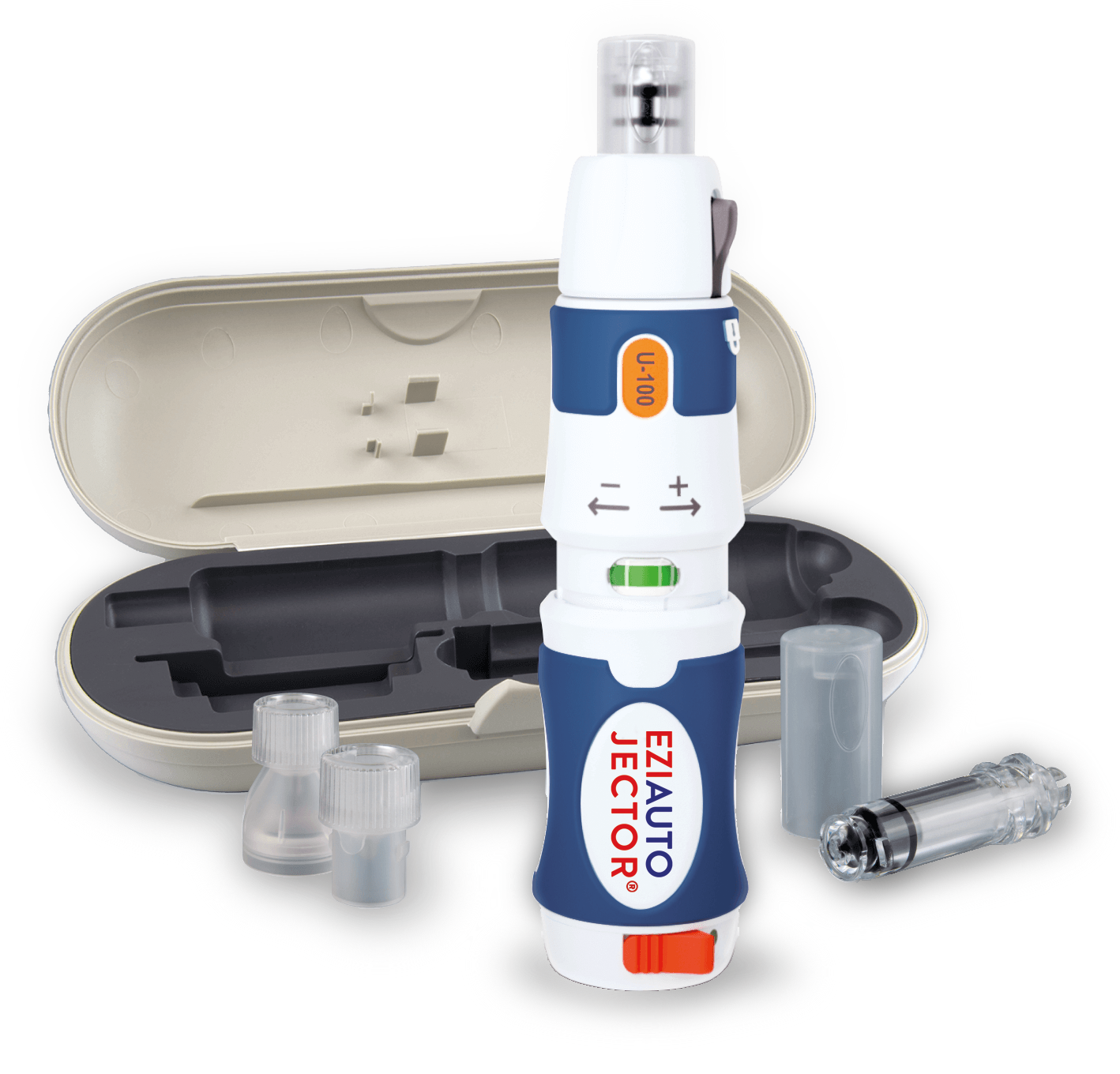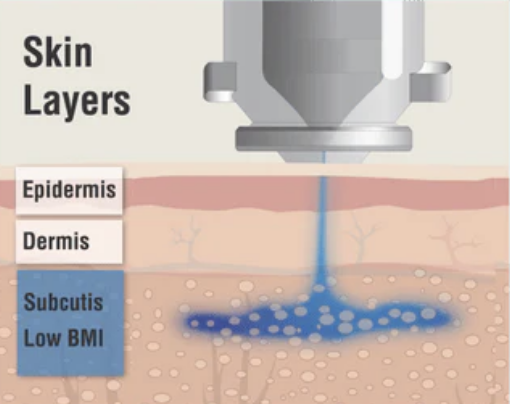
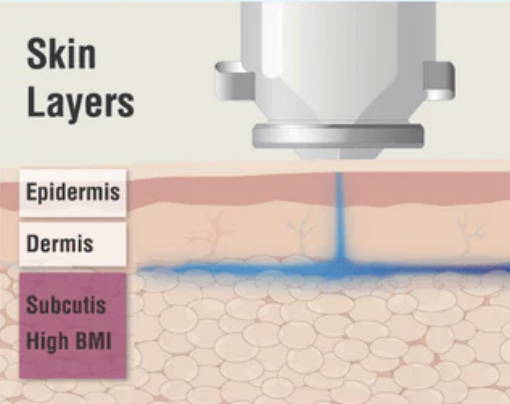
Needle-Free Insulin Administration
The EziAutoJector® utilises advanced high-pressure jet administration technology to deliver medication through a micro-orifice in a specially engineered nozzle.
This high-velocity stream penetrates the skin without the use of a needle, delivering insulin directly into the subcutaneous tissue. Once administered, the liquid follows the path of least resistance, enabling even distribution and effective absorption.
This needle-free approach enhances user comfort—particularly for individuals with needle-related anxiety—and supports more efficient insulin uptake, promoting better treatment outcomes and a more empowering experience in diabetes care.
One size fits all
The EziAutoJector® is designed to be suitable for individuals of all sizes, genders, and ages—delivering consistent performance across a broad range of users.
The advanced jet-administration technology may be particularly beneficial for individuals with a higher Body Mass Index (BMI), ensuring effective insulin delivery without the need to adjust needle length or technique.
Get started with EziAutoJector® needle-free delivery device.

ISO 13485

CE CERTIFIED

NHS COVERED
Disclaimers:
01. Compatible insulin types: Human and animal; Fast acting insulins (bolus); Rapid Acting Insulin Analogues; Regular Human Insulin; Basal insulins; Intermediate-acting, commonly: NPH/ Isophane; Long-acting, commonly: Glargine, detemir, degludec; Premixtures (e.g. 30/70).
02. In a user survey, 78% of respondents answered they would recommend to others.
03. In a user survey, 40% of the respondents answered they noticed using less insulin.
04. Needle-Free Jet Injection of Rapid-Acting Insulin Improves Early Postprandial Glucose Control in Patients With Diabetes. Diabetes Care, October 2013.
05. Body mass index and the efficacy of needle-free jet injection for the administration of rapid-acting insulin analogues, a post hoc analysis. Diabetes, Obesity and Metabolism, July 2012.
06. Improved Pharmacokinetic and Pharmacodynamic Profile of Rapid-Acting Insulin Using Needle-Free Jet Injection Technology. Diabetes Care, August 2011
07. A Pilot Study to Examine the Tolerability and Device Preference in Type 1 Diabetes of Insulin Apart Administered by Needle-Free Technology compared with Subcutaneous Injection. Diabetes Technology & Therapeutics, 2014.
08. See medical deck Cost Analysis – North America, 2022 available on the Partner page.
09. Calculated at 4 injections a day with a hypodermic needle. 4 injection a day, every day of the year, adds up to 1,460 needles a year saved from use.
*. The Needle-free Device is calibrated for U-100 insulins
1. **. Needle gauge 26-27 is commonly used for subcutaneous injections and is around 0.40 – 0.45mm in diameter. The orifice of the Needle-Free Device Nozzle is about 0.15mm in diameter which is much smaller than conventional needle gauges used for subcutaneous insulin injections.
***. https://pubmed.ncbi.nlm.nih.gov PMID:18820853